Introduction to CH-NMR-NP system
The 13C/1H-NMR database for natural products [CH-NMR-NP] is mainly composed of natural products that were published in major journals in the years between 2000 and the spring of 2014.
For a natural product to be included in the database, complete 13C-NMR data was a strict precondition.
No such precondition was set for 1H and as a result some compounds show incomplete 1H-NMR data.
As it concerns a database, CH-NMR-NP lacks completeness.
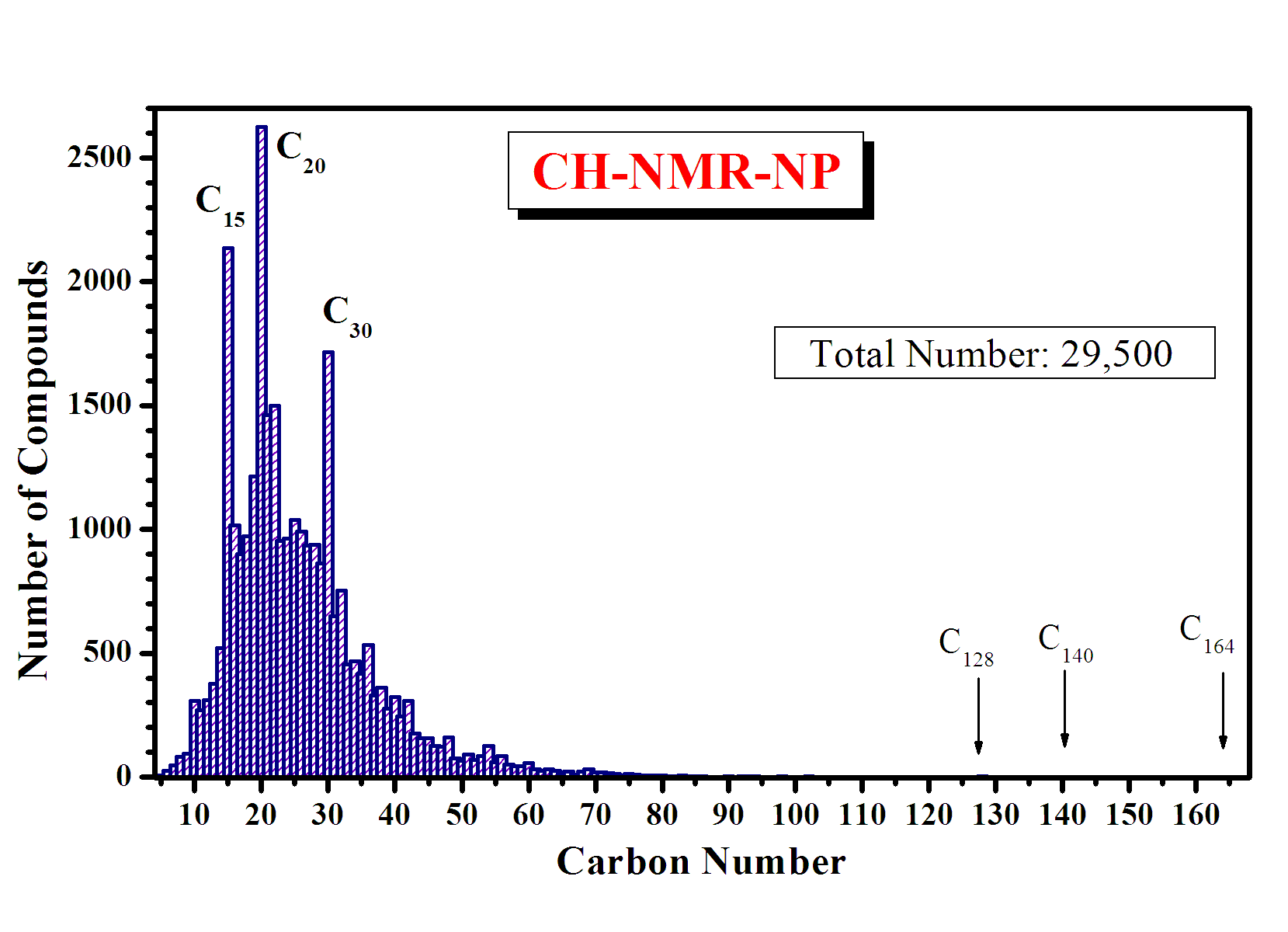
The number of the compounds in natural products compiled from the published papers is about 29,500.
Additionally, about 6,000 organic compounds with 1H/13C spectral patterns were compiled from SDBS-NMR, and the total number of the compounds is approximately 35,500.
©Kikuko Hayamizu
NMR Database Search
Search Results
Welcome to CH-NMR-NP
Introduction
CH-NMR-NP is a 13C and 1H NMR database of organic natural products compiled from published papers. The building began in May, 2002 and completed in 2014 summer to check the data. The data are mainly compiled from papers in the following research journals issued mainly from 2000 to spring in 2014. With addition of about 6,000 compoundst from SDBS-NMR, which include 1H and 13C spectral patterns observed. The total number of the data included in CH-NMR-NP is 35,500.
Journals from which data were compiled
- Chem. Pharm. Bull. 2000-2013
- J. Antibiotics 2000-2013
- Tetrahedron + Tetrahedron Letters 2000-2014 spring
- J. Natural Products 2000-2014 spring
- Phytochemistry + Phytochem. Lett. 2000-2013
- J. Org. Chem. + Eur. J. Org. Chem. 2000-2013
- Magn. Reson. Chem. 1996-2013
- Organic Letters 1999-2014 spring
- Other Journals
The principles of data inclusion
- The chemical shift tables of 1H and 13C data were described.
- The chemical structure was clearly drawn.
- The name and molecular formula (consistent with the chemical structure) were given.
- No obvious errors were found in the original literature source.
- The compounds were judiciously selected so as not to overlap similar compounds with similar NMR spectra.
As the results, the database does not include every compound that appeared in the above journals.
Chemical Structure with Assignment
- Chemical structure was drawn by using an ISIS_Draw.
- The numbering at the carbon positions corresponds to the 1H and 13C chemical shifts.
- To obtain 1:1 correspondence between the carbon position and the chemical shift, the different numbers are given for each carbon except for equivalent carbons.
- When the carbons in a long alkyl chain give the same chemical shifts, the same number may be given.
- For numbering the carbons, alphabets, Greek characters, or symbols like 1' and 1'' are all converted to figures. The order of the numbering is followed to the authors.
NMR Spectral Data
For each carbon in the chemical structure, following data are given.
- 13C Chemical shift.
- Number of hydrogen atoms attached ( C, CH, CH2 and CH3 )
- 1H Chemical shift.
- 1H Splitting information and additionaly proton types such as OH, NH, NH2 and so on.
- 13C Assignment exchangeable, if any.
- Number of equivalent carbons.
Compound Information
- "Name" is the compound name given by the authors in the literature.
- "Molecular Formula" is molecular formula derived from the chemical structure of ISIS/Base.
- "Molecular Weight" is the molecular formula calculated from the chemical structure by ISIS/Base.
- "Characteristic" is taken from the description of the literature to classify the natural compound.
- "Chemical Name" is the full chemical name in the literature including IUPAC name.
- "CAS Registry No." is CAS registry number.
NMR Measurement Conditions
- "Solvent" is the solvent for the measurement. If the solvent is not described, the data is empty. The specified conditions of temperature or pH are included.
- "1H Frequency (MHz)" is the measuring frequency for 1H NMR spectrum.
- "Shift Ref" is the chemical shift references for 1H and 13C spectra given by authors. TMS is the internal reference of tetramethylsilane. The solvent shifts for the references were given in ppm unit for "1H / 13C" shifts.
Others
- "NP No." is given for an organic natural compound. The same number is given if the spectra were measured by different solvents or different conformers.
- "Spectral Key" is the spectral key for carbon and proton NMR spectra. When the measuring conditions (like different solvents, temperatures and conformers) were different, a compound may have two or more Spectral keys.
- "Source" is the origins of the compound taken from.
- "Remarks" are taken from the authors' description concerning "Source".
- "Reference" is the literature from which the data are compiled.
- "Comments" are the compiler's comments.
- "Clear Errors in Assignment" may be corrected by compilers.
The distribution, by the carbon number, of the organic natural compounds compiled in CH-NMR-NP is shown in the graph below.
Organic compounds from SDBS-NMR
About 6,000 organic compounds are included from SDBS-NMR. The C-numbers are from 1 to 48 and about 70 % compounds are composed of C5 to C15, mainly from commercial sources. Most of the 1H spectra were observed at 400 MHz. Since the digital spectral patterns were compiled, the view and download the spectral patterns are possible.
Inquiry form
Please feel free to fill out the form below to register your request for NMR database “CH-NMR-NP”.Thank you for your request.
We’ve received your request for information about the Natural Product NMR-DB “CH-NMR-NP”.UNDER MAINTENANCE
We sincerely apologize for the inconvenience.Natural Product NMR-DB "CH-NMR-NP" is currently undergoing maintenance.
We should be back shortly. Thank you for your patience.



